AAPE / ADSC-CM Treatment Is Derived From Adipose (Fat) Stem Cells
The use of AAPE, “fat” or adipose-derived stem cells conditioned media or ADSC-CM has been used successfully in the treatment of female hair loss. The non-invasive treatment is described in the following journal article, including a new study and scientific analysis of hair growth measurements as well as photos of the results, as published in the International Journal of Dermatology. This advanced stem cell-derived protein extract called AAPE can now be applied in a clinical setting without discomfort or downtime as a lunch-hour session at Bauman Medical Hair Transplant and consultation or more information.
Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study
Hyoseung Shin1, MD, Hyeong Ho Ryu2, MD, Ohsang Kwon2, MD, PhD, Byung-Soon Park3,*, MD, PhD, and Seong Jin Jo1,*, MD, PhD
1Department of Dermatology, Dongguk University Ilsan Hospital, Goyang, South Korea, 2Department of Dermatology, Seoul National University College of Medicine, Seoul, South Korea, and 3Cellpark Clinic, Seoul, South Korea
*These authors contributed equally to this work.
Conflicts of interest: None.
International Journal of Dermatology 2015, 54, 730–735 – International Society of Dermatology
Abstract
Background: Female pattern hair loss (FPHL) is a common disorder but presents severe psychosocial problems in many female patients. Adipose tissue-derived stem cells (ADSCs) and conditioned media of ADSCs (ADSC-CM) are reported to promote hair growth in vitro. However, there are no clinical reports on the treatment of alopecia using ADSC-CM.
Objectives: This study evaluates our clinical experience in the use of ADSC-CM for the treatment of FPHL.
Methods: A retrospective, observational study of outcomes in 27 patients with FPHL treated with ADSC-CM was performed. To evaluate the efficacy of the treatment, patients’ medical records and phototrichographic images were analyzed.
Results: The application of ADSC-CM showed efficacy in treating FPHL after 12 weeks of therapy. Hair density increased from 105.4 to 122.7 hairs/cm2 (P < 0.001). Hair thickness increased from 57.5 lm to 64.0 lm (P < 0.001). None of the patients reported severe adverse reactions.
Conclusions: The application of ADSC-CM is a potential treatment option for FPHL.
Introduction
Stem cells are immature precursor cells characterized by capacities of self-renewal and multi-lineage differentiation. Mesenchymal stem cells (MSCs), which represent a type of stem cell, were first identified in bone marrow, but the existence of MSCs in connective tissue has been reported.1 It was recently found that MSCs are abundant in adipose tissue, which is easily accessible. The yield of MSCs from adipose tissue is approximately 40-fold greater than that from bone marrow.2 Such characteristics can make it easy to investigate and use MSCs in clinical fields. Adipose tissue-derived stem cells (ADSCs) produce growth factors that have paracrine effects on surrounding cells. These growth factors include vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and others.3 Growth factors have various functions. In a transgenic mouse model, VEGF has been proven to affect hair growth and follicular size by angiogenesis.4 Hepatocyte growth factor and IGF also activate hair growth through various pathways.5,6 Platelet-derived growth factor induces and maintains anagen hair in murine hair follicles.7 These observations suggest that ADSCs may have a therapeutic effect on hair loss. Indeed, we previously reported that ADSCs and the conditioned media of ADSCs (ADSC-CM) promote hair growth in vitro.8,9
Female pattern hair loss (FPHL) is a very common type of alopecia seen in women. Its prevalence is approximately 6% in women aged under 50 years and 38% in women aged 70 years and over.10 Although FPHL rarely progresses to the total hair loss seen in male androgenetic alopecia, hair loss is more distressing to women than men because women with FPHL have a more negative body image in comparison with balding men.11 Nevertheless, FPHL patients have fewer therapeutic options than male androgenetic alopecia patients. Lower percentage topical minoxidil is the only medication approved by the US Food and Drug Administration for FPHL. Anti-androgen drugs and topical estrogen are used for the treatment of FPHL. However, they do not always achieve successful results. Anti-androgens and finasteride carry a risk for the feminizing of a male fetus. These treatments are therefore not safe in reproductive women. Topical estrogen still lacks convincing clinical trials.12 There is still a significant need for more effective therapy for FPHL. This study reports on our evaluation of outcomes in 27 patients in whom ADSC-CM was used to treat FPHL.
Materials and methods
Subjects
The medical records of FPHL patients were reviewed to collect subjects treated with only ADSC-CM at the dermatologic clinic (Cellpark Clinic, Seoul, South Korea) from January to October 2012. We excluded subjects who had used any products or drugs that might have influenced hair growth during the six months prior to ADSC-CM therapy. The patients had all been informed about the treatment they would receive. This study was approved by the Institutional Review Board of Seoul National University Hospital.
Materials
The authors used the commercial ADSC-CM product AAPE (now known as NGAL) (Prostemics Research Institute, Sungnam, South Korea). AAPE is produced from human subcutaneous adipose tissue that is obtained by medical liposuction from healthy persons who provide informed consent. AAPE is manufactured by the method described below.9 Adipose tissues were exposed to 0.075% type II collagenase (Sigma-Aldrich Corp., St Louis, MO, USA) for 30 minutes at culture temperature, centrifuged at 400 g for 10 minutes, and washed and resuspended in phosphate-buffered saline (PBS). The stromal cell fraction was filtered through a 70-lm cell strainer (BD Biosciences, Inc., San Jose, CA, USA). Using Histopaque-1077 (Sigma-Aldrich Corp.), ADSCs were isolated from the filtrate and cultured at 37 °C in 5% carbon dioxide in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Characteristic expression levels of stem cell-related surface markers were confirmed by flow cytometry. ADSCs expressed CD73, CD90, and CD105 and were lacking in CD34 and CD49d. Adipogenic, osteogenic, and chondrogenic differentiation was also checked by the conventional method.13 After the isolation of ADSCs, the cells were pooled. ADSCs were cultured and expanded in normal control medium and used in experiments at passage 4. Cells were finally frozen in aliquots using Cell FreezerTM (Genenmed, Inc., Seoul, South Korea) for future use. A frozen vial containing 1 9 106 cells was inoculated into culture medium containing 10% FBS. After repeated subculture to reach a density of 5 9 108 cells, the expanded ADSCs were introduced into Cell FactoryTM CF10 (Nalge Nunc International Corp., Rochester, NY, USA) in DMEM/F12 serum-free medium (WelGene, Inc., Taegu, Korea). Cultures were conducted under hypoxic conditions by providing 2% oxygen using a nitrogen gas supply in a humidified multichannel incubator for two weeks. The conditioned media were collected and microfiltered and total protein production quantitated. Finally, for fresh use, 4-ml vials containing equal protein concentrations were freeze-dried as a single lot sample preparation of AAPETM.
Treatment protocol
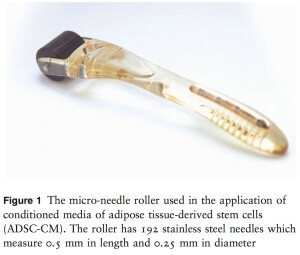
The authors set a 12-week protocol for the treatment of FPHL with ADSC-CM. The treatment course consisted of repeated applications of ADSC-CM once per week for 12 consecutive weeks. The scalp area was gently cleansed prior to the application of AAPE with a micro-needle roller (MRS-05; Union Medical Co. Ltd, Uijeongbu, South Korea) (Fig. 1). Routine phototrichographic images were taken of all patients at the first visit and at 12 weeks using a digital camera (Folliscope; Lead M Co. Ltd, Seoul, South Korea) at magnifications of 930 and 960. At the first visit, the area in the midline scalp was tattooed with a black dot, and a phototrichogram was taken with the dot positioned in the center of the image. A phototrichogram of the same area was taken after 12 weeks of treatment.
Measurements and statistical analysis
Hair density (hair count per cm2) was calculated by counting the total number of hairs in the target area. Hair thickness was calculated as the average diameter of hairs measured manually on the phototrichograms. The Kolmogorov–Smirnov test was used to evaluate whether the data showed a normal distribution. Subsequently, the Wilcoxon signed rank test was carried out to evaluate changes in hair density and hair thickness. Spearman’s correlation analysis was used to assess correlations between patient age and treatment responses such as increases in hair thickness and hair density. P-values of < 0.05 were considered to indicate statistical significance.
Figure 1 The micro-needle roller used in the application of conditioned media of adipose tissue-derived stem cells (ADSC-CM). The roller has 192 stainless steel needles which measure 0.5 mm in length and 0.25 mm in diameter
Results
The number of FPHL patients treated with only ADSC-CM was 27. All patients completed the 12-week treatment course. The mean ` standard deviation (SD) patient age was 41.9 ` 13.4 years (range: 22–69 years). Hair loss in all patients was classified as Ludwig type I. The onset of hair loss as reported by the patients had occurred at a mean ` SD of 6.4 ` 0.9 years (range: 1– 22 years) previously. All patients were healthy and without other current medical problems. None of the patients had used any products or taken any drugs that might influence hair growth for at least six months prior to the start of this study. There were no pregnant or lactating women among the subjects.
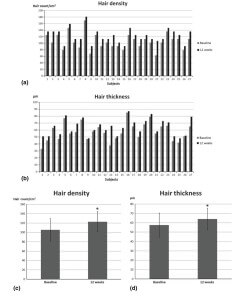
Figure 2 Data on hair growth in 27 women with female pattern hair loss at baseline and at 12 weeks after the initiation of treatment with conditioned media of adipose tissuederived stem cells (ADSC-CM), showing (a) hair density in all subjects, (b) hair thickness in all subjects, (c) mean hair density, and (d) mean hair thickness. (Data are mean +/- standard deviation; *P < 0.001)
Mean hair density increased from 105.4 to 122.7 hairs/ cm2 over the 12 weeks of treatment (P < 0.001) (Fig. 2a, c), representing an increase of 16.4%. Mean hair thickness increased from 57.5 lm to 64.0 lm (P < 0.001) (Fig. 2b, d), an increase of 11.3%. The application of ADSC-CM for 12 weeks induced statistically significant improvements in hair density and hair thickness. There was no correlation between patient age and an increase in hair thickness or density (age and hair thickness, P = 0.706; age and hair density, P = 0.342). Neither was the duration of hair loss correlated with treatment response (disease duration and hair thickness, P = 0.584; disease duration and hair density, P = 0.128). No severe adverse effects were seen in any patient. No patient reported irritation or itching. The only inconvenience reported referred to the pricking of the needles during application; however, nobody dropped out of the treatment course as a result of this pain.
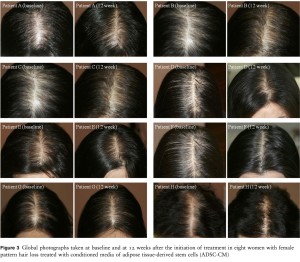 Figure 3 Global photographs taken at baseline and at 12 weeks after the initiation of treatment in eight women with female pattern hair loss treated with conditioned media of adipose tissue-derived stem cells (ADSC-CM)
Figure 3 Global photographs taken at baseline and at 12 weeks after the initiation of treatment in eight women with female pattern hair loss treated with conditioned media of adipose tissue-derived stem cells (ADSC-CM)
Discussion
Won et al.14 showed that ADSC-CM treatment enhanced the proliferation of cultured human dermal papilla cells (DPCs) by up to 130%. This result was explained by the activation by ADSC-CM of both Erk and Akt signaling pathways. The Akt signaling pathway mediates survival signals, and the Erk signaling pathway plays a role in mitogenesis. Both enhance the survival and proliferation of DPCs.15,16 In addition, ADSC-CM modulates the cell cycle of DPCs. Cyclin D1 and CDK2, key cell cycle-related molecules, are upregulated by ADSC-CM.14 These results are meaningful with regard to hair growth because dermal papilla size is known to correlate with the hair growth cycle, and the number of DPCs increases in the anagen phase.17
In a previous ex vivo study, the length of the hair follicles in a group of patients treated with ADSC-CM significantly increased by 40%, as much as that seen in patients treated with 1 mM of minoxidil for six days.14 In the same study, hair growth was stimulated by ADSCCM in C3H/HeN nude mice. ADSC-CM had an effect in inducing the anagen phase of murine hair.14 These results suggest ADSC-CM may represent a promising therapeutic option in hair loss disorders such as FPHL.
Furthermore, the effectiveness of ADSCs in hair growth promotion can be potentiated through hypoxic conditions, in which ADSCs produce more growth factors such as VEGF, PDGF, and IGF binding protein (IGFBP).9 As described earlier, VEGF is related to hair growth.4 PDGF and its receptors play important roles in follicular development.7 IGFBPs modulate IGF-1 in hair regeneration. Co-treatment with IGF-1 and IGFBPs enhances hair regeneration.18 Park et al.9 showed that ADSC-CM produced in hypoxic conditions induced the anagen phase more rapidly than in normoxic conditions. AAPE is a commercialized ADSC-CM product produced under hypoxic conditions by the provision of 2% oxygen. AAPE is approved by the Korean Food and Drug Administration. Based on evidence from previous experiments, the present authors applied it in the treatment of FPHL with caution. We chose to use it in women rather than in men because treatment options for FPHL and their efficacy are more limited than are those for male androgenetic alopecia. To the best of our knowledge, this is the first report of the clinical use of ADSC-CM for FPHL.
A reduction in hair density and thickness involving the mid-line scalp is a typical feature of FPHL. Hair density and thickness are objective parameters with which to demonstrate the severity of FPHL.19 All 27 patients in the present series showed significant improvements in both hair density and thickness in phototrichograms, although the clinical improvement was rather slight (Fig. 3).
The present study represents a retrospective case series and did not include a control group. Therefore, the interpretation of its findings may be limited. Nevertheless, the changes in hair density and thickness were statistically significant and may be compared with those in previous controlled studies of FPHL if demographic differences are taken into account.
Lucky et al.20 revealed that hair density was increased by 17.3% by the application of 5% minoxidil twice per day for 48 weeks and by 13.8% by 2% minoxidil applied twice per day for 48 weeks in FPHL patients. In our study, hair density was increased by 16.4% by one application of ADSC-CM per week for only 12 weeks. Thus, ADSC-CM was more effective than 2% minoxidil within a shorter period in terms of the percentage improvement. Price and Menefee21 showed that hair thickness increased by 5.0% in FPHL patients who applied 2% minoxidil twice per day for 24 weeks. This outcome was observed in FPHL patients with hair loss of Ludwig types I and II, whereas all patients in the present series had Ludwig type I hair loss. Although there is a difference in the severity of FPHL between subjects in the earlier study21 and those in the present series, it seems promising that we were able to observe an 11.3% increase in hair thickness attributable to the application of ADSC-CM for 12 weeks.
We used micro-needles to deliver ADSC-CM to the scalp in our clinic; therefore, all patients were required to visit the clinic once per week. Although this can be perceived as annoying, such a protocol has some advantages. Applications of ADSC-CM are performed with precision just once per week by medical staff, whereas topical minoxidil should be applied twice per day. It is well known that it is very difficult to keep applying topical minoxidil over a long period. Mapar and Omidian22 reported that almost all patients among 1495 men with androgenetic alopecia gradually discontinued the treatment earlier than expected. As ADSC-CM contains ingredients of large molecular sizes, a technique that enhances transdermal delivery should be incorporated into the treatment.23 We used the micro-needle roller, which is widely used to enhance the absorption of topical agents. To our knowledge, there is no evidence that the application of the micro-needle itself ameliorates hair loss.24
With regard to safety, no noticeable adverse effects that infringe safety regulations were reported. None of the patients felt any discomfort other than a pricking sensation when the micro-needles were applied. There were no dropouts during the 12-week treatment course.
We were unable to quantify patients’ subjective satisfaction through the medical record review. The clinical photographs were not standardized for objective evaluation. It is regrettable that we are unable to include such clinical data. This study identified only the short-term, 12-week effects of ADSC-CM. Nine of the 27 patients were followed up for six months, and one was followed up to one year. The clinical improvement was maintained in these patients, none of whom experienced aggravated hair loss after the completion of treatment. These findings were confirmed by objective measurements of hair density and thickness (data not shown). However, the condition of the 17 patients who were not followed up is unknown. Despite these limitations, we believe that ADSC-CM represents a promising therapeutic strategy and an alternative treatment for FPHL, as shown by objective assessment with phototrichography. We believe that this work provides useful pilot data with which to support the instigation of a randomized controlled trial to establish the efficacy of ADSC-CM in the treatment of FPHL.
References
1 Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006; 99: 1285–1297.
2 Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24: 1294– 1301.
3 Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004; 94: 678–685.
4 Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest 2001; 107: 409–417.
5 Jindo T, Tsuboi R, Takamori K, et al. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol 1998; 110: 338–342.
6 Weger N, Schlake T. IGF-1 signaling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol 2005; 125: 873–882.
7 Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci 2006; 43: 105–115.
8 Won CH, Yoo HG, Park KY, et al. Hair growth- promoting effects of adiponectin in vitro. J Invest Dermatol 2012; 132: 2849–2851.
9 Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res 2010; 31: 27–34.
10 Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol 2001; 144: 297–304.
11 Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol 1993; 29: 568–575.
12 Blume-Peytavi U, Kunte C, Krisp A, et al. Comparison of the efficacy and safety of topical minoxidil and topical alfatradiol in the treatment of androgenetic alopecia in women. J Dtsch Dermatol Ges 2007; 5: 391–395.
13 Kim WS, Park BS, Kim HK, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 2008; 49: 133–142.
14 Won CH, Yoo HG, Kwon OS, et al. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci 2010; 57: 134–137.
15 Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 1997; 9: 180–186.
16 Tang Y, Zhou H, Chen A, et al. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem 2000; 275: 9106–9109.
17 Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol 1999; 113: 873– 877.
18 Weger N, Schlake T. IGFBP3 modulates cell proliferation in the hair follicle. J Invest Dermatol 2005; 125: 847– 849.
19 Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977; 97: 247–254.
20 Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol 2004; 50: 541– 553.
21 Price VH, Menefee E. Quantitative estimation of hair growth. I. androgenetic alopecia in women: effect of minoxidil. J Invest Dermatol 1990; 95: 683–687.
22 Mapar MA, Omidian M. Is topical minoxidil solution effective on androgenetic alopecia in routine daily practice? J Dermatolog Treat 2007; 18: 268–270.
23 Park BS, Jang KA, Sung JH, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 2008; 34: 1323–1326.
24 Lee YB, Eun YS, Lee JH, et al. Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol 2013; 40: 81–83.
If you or someone you know has hair loss, hair thinning, baldness, or eyebrow / eyelash concerns, click to start either a long-distance virtual consultation OR an in-person, in-office consultation with Dr. Bauman. You can also Ask Dr. Bauman a Question or simply call Bauman Medical Group at +1-



 Nanofat – ALMI Autologous Lipocyte Micronized Injection for Hair Regrowth
Nanofat – ALMI Autologous Lipocyte Micronized Injection for Hair Regrowth Frequently Asked Questions About PRP For Hair Regrowth FAQs
Frequently Asked Questions About PRP For Hair Regrowth FAQs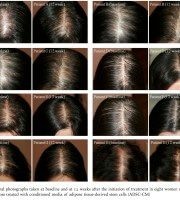 New Study: Use of AAPE Adipose-Derived Stem Cell Conditioned Media ADSC-CM in Female Pattern Hair Loss
New Study: Use of AAPE Adipose-Derived Stem Cell Conditioned Media ADSC-CM in Female Pattern Hair Loss Dr. Alan J. Bauman, M.D.Hair Loss & Hair Transplant ExpertBoca Raton, FL
Dr. Alan J. Bauman, M.D.Hair Loss & Hair Transplant ExpertBoca Raton, FL






