Exciting Breakthrough in Hair Loss Treatment: GT20029 Emerges as a Promising Topical Option for Androgenetic Alopecia
Hello, I’m Dr. Alan Bauman, founder and medical director of Bauman Medical, a leading hair restoration clinic in Boca Raton, Florida. For over 25 years, I’ve dedicated my practice to helping patients combat hair loss through innovative, non-invasive treatments. Today, I’m thrilled to share some exciting developments in the world of hair restoration: the promising results from Phase II clinical trials of GT20029, a first-in-class topical drug for androgenetic alopecia (AGA), commonly known as male pattern baldness or female pattern hair loss. This could represent a game-changer for those seeking effective, low-risk options beyond traditional therapies.
As we approach the end of 2025, advancements like GT20029 remind us that hope is on the horizon for millions affected by hair thinning. In this article, I’ll break down what GT20029 is, how it works, its recent trial outcomes, how it stacks up against current treatments, and what this means for you as a patient. Let’s dive in!
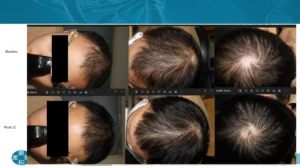
Image: Before-and-after results from GT20029 Phase II clinical trial. Credit: Courtesy of Kintor Pharmaceutical, shared via interview with Reddit user noeyys .
What is GT20029 and Why Is It Generating Buzz?
GT20029 is an investigational topical medication developed by Kintor Pharmaceutical, a biopharma company focused on androgen-related conditions. Unlike oral drugs that affect your entire body, GT20029 is applied directly to the scalp, targeting the root cause of AGA: overactive androgen receptors in hair follicles.
Androgenetic alopecia affects about 50 million men and 30 million women in the U.S. alone, leading to progressive hair thinning due to dihydrotestosterone (DHT), a hormone that shrinks hair follicles over time. GT20029 uses a cutting-edge technology called PROTAC (proteolysis-targeting chimera), which essentially “tags” and degrades these problematic androgen receptors right at the source – your scalp’s hair follicles. This localized approach aims to promote hair regrowth without the systemic side effects often seen with other treatments.
Recent announcements from Kintor highlight that GT20029 has successfully completed Phase II trials for hair loss, with data published in December 2025 showing significant improvements in hair density.[2][6] It’s now advancing toward Phase III trials, which could bring us closer to FDA approval in the coming years.[5] This is particularly exciting because it’s designed for twice-weekly application, making it convenient for busy lifestyles.
Understanding the Mechanism of Action of GT20029
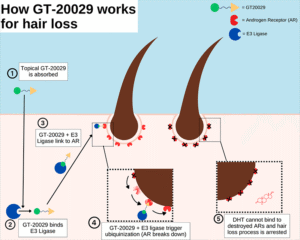
At its core, GT20029 operates through a novel proteolysis-targeting chimera (PROTAC) mechanism, which selectively degrades androgen receptors (AR) in the hair follicles. When applied topically to the scalp, GT20029 binds to the AR proteins and recruits an E3 ubiquitin ligase, marking the receptors for destruction by the cell’s natural proteasome system.[0][2][4] This degradation process effectively blocks the harmful effects of androgens like DHT, which would otherwise bind to these receptors and trigger follicle miniaturization leading to hair loss. Unlike traditional anti-androgen treatments that merely block receptors or inhibit hormone production systemically, GT20029’s targeted degradation offers a more precise, localized intervention, minimizing off-target effects and potentially improving safety for long-term use.[1][5][6] For patients, this means addressing the root cause of AGA at the cellular level without disrupting overall hormone balance, making it a promising option for those wary of broader hormonal impacts.
Key Findings from the Phase II Clinical Trials
In a multicenter, randomized, double-blind study involving 180 men with mild-to-moderate AGA, GT20029 was tested at different doses and frequencies over 12 weeks. The results, freshly released this month, are encouraging:
- Hair Regrowth: Participants saw an average increase of up to 16.8 hairs per square centimeter in the target area – a statistically significant improvement over placebo.[1][8] Hair width also improved, leading to thicker, fuller-looking hair.
- Best Results in Specific Groups: Younger patients, those with moderate hair loss, or individuals new to treatments responded particularly well. This suggests GT20029 could be ideal for early intervention.
- Convenience Factor: The twice-weekly dosing (at 1.0% concentration) performed as well as daily applications, which could boost adherence and long-term success.
These outcomes build on earlier Phase I data, confirming low absorption into the bloodstream and minimal impact on overall hormone levels.[0] At Bauman Medical, we always emphasize early detection and treatment – remember, time equals follicles! Starting therapies like this sooner could preserve more of your natural hair.
How Does GT20029 Compare to Current Hair Loss Therapies?
For context, let’s compare GT20029 to established treatments I often recommend at my clinic:
- Minoxidil (Rogaine®): This over-the-counter topical solution stimulates blood flow to follicles and is effective for many, but it requires daily use and can cause scalp irritation. GT20029 targets the androgen pathway more directly, potentially offering better results for DHT-driven hair loss without the messiness of foams or lotions.
- Finasteride (Propecia®): An oral DHT blocker that’s FDA-approved and proven to halt progression in about 86% of men. However, some patients worry about side effects like reduced libido (affecting 1-2%). GT20029’s topical, receptor-degrading mechanism could avoid these by staying localized, making it a safer alternative for those concerned about systemic effects.
- Dutasteride: Similar to finasteride but stronger, often used off-label. Again, oral delivery raises similar concerns. GT20029 might fill the gap for patients seeking non-oral options.
- Advanced Therapies at Bauman Medical: We offer combinations like low-level laser therapy (e.g., Bauman TURBO LaserCap), PRP (platelet-rich plasma) injections, Exosome Therapy, and our proprietary PEPgro™ peptide therapy. GT20029 could complement these, especially for patients with androgen-sensitive hair loss. In fact, emerging drugs like this align with our multi-therapy approach to maximize regrowth.
While GT20029 isn’t available yet, its profile suggests it could become a first-line topical treatment, especially for those avoiding pills. Early data shows efficacy comparable to or better than some existing options in short-term studies, with fewer applications needed.[1]
Safety Profile: What Patients Need to Know
Safety is paramount in hair restoration. GT20029 was well-tolerated in trials, with side effects mostly mild and limited to the scalp – things like temporary itching or redness in about 7% of users.[2][8] Importantly, there were no reports of sexual dysfunction, hormonal changes, or serious adverse events, which sets it apart from systemic drugs.
Of course, as with any new treatment, we’ll await Phase III results for long-term data. At Bauman Medical, we monitor these developments closely to ensure our patients get safe, evidence-based care.
When Will GT20029 Be Available?
As of December 2025, GT20029 is progressing to Phase III trials in China and potentially the U.S. soon.[3][7] Approval could take 2-5 years, but interested patients can explore clinical trial opportunities on ClinicalTrials.gov. In the meantime, schedule a consultation at Bauman Medical for personalized plans using today’s proven therapies.
Final Thoughts: A Brighter Future for Hair Loss Sufferers
GT20029 represents the innovative spirit driving hair restoration forward – targeted, convenient, and patient-friendly. While it’s not a cure-all, its potential to degrade androgen receptors without body-wide effects could help countless individuals regain confidence. If you’re experiencing hair loss, don’t wait – early action is key.
At Bauman Medical, we’re here to guide you through options like this. Contact us today for a virtual or in-person consultation. Remember, your hair’s future starts now!
Stay informed and hopeful,
Dr. Alan J. Bauman, MD, ABHRS, FISHRS
Founder & CEO, Bauman Medical
References:
- First-in-Class Topical GT20029 Demonstrates Promising Phase 2 Efficacy and Tolerability for AGA
- Efficacy and safety of topical GT20029 in male patients – PubMed
- GT-20029 – Wikipedia
- Kintor Pharma Advances to Phase III with GT20029
- Full article: Efficacy and safety of topical GT20029
- (PDF) Efficacy and safety of topical GT20029
- Kintor Pharmaceutical Limited – Official Site
- Time Equals Follicles: The Evolving Landscape of Hair Loss Treatment with Alan J. Bauman, MD
- GT20029: A Potential Breakthrough Treatment for Androgenic Alopecia
- Kintor’s GT20029 IND for PROTAC AR Degrader Was Accepted by NMPA
- Kintor Pharmaceutical Receives IND Clearance by the U.S. FDA for GT20029
- Kintor Advances With GT20029 Clinical Trials for Hair Loss